The Kjeldahl determination method is a classical method for the determination of protein content in foods. The results are stable and accurate. However, the operation is cumbersome, the sample digestion takes time and trouble, and the SO2 emitted during digestion directly jeopardizes the health of the operator. The automatic protein analyzer is expensive and is still difficult to popularize. This article uses KDY-04 (A) nitrogen determination instrument to determine the protein content of food, the results and the classic Kjeldahl method has no statistically significant difference, and the operation is simple, the result is accurate, the analysis speed, eliminate the air pollution, available Determination of protein in bulk samples. Related equipment: Container weight meter Amylose content meter Graphite Box,Graphite Gear Box,Molded Graphite Box,Parts For Graphite Gearbox Henan Carbon Road New Material Technology Co., Ltd , https://www.kymoldedgraphite.com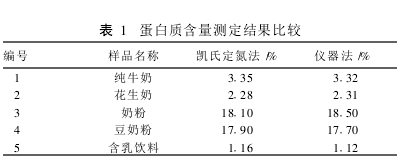
1 Materials and Methods
1. 1 Instruments and reagents KDY-04 (A) nitrogen determination instrument; sample device: 4-well digester; hydrochloric acid standard solution: c (HCI) = 0,0500mol / L; sulfuric acid (Ï20 = 1 84g / ml); Sodium hydroxide solution (400g/L); boric acid solution (20g/L); indicator solution: 1 part methyl red ethanol solution (1g/L) and 5 parts bromocresol green ethanol solution (1g/L), when in use Mixed; Catalyst: selenium powder or potassium sulfate + copper sulfate (10+1); hydrogen peroxide (30%).
1·2 Measurement method
1·2·1 Sample Digestion Accurately weigh 0·5~2·0g of mixed solid sample or absorb 5·0~10·0ml liquid sample into clean digestive tract, add 0·5~1·0g selenium powder or 10g Potassium sulphate + 1g copper sulfate, 10ml sulfuric acid, shake, add 10ml hydrogen peroxide, mix, put the digestive tube into the digester, put the drain pipe on the seal ring, open the suction three-way water, make the pumping The three-way steam is in suction. Turn on the power supply, digest, and if the sample of powdered milk is indigestible, increase the amount of hydrogen peroxide added to the digestion process, until the digestive fluid is transparent blue-green liquid, turn off the power, cool, and set the volume to 100ml. This process takes about 1~2h. 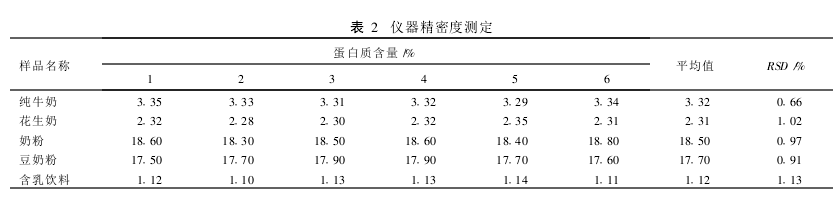
1ã€2・2 Measure Turn on the power and follow the instruction of the instrument to debug the instrument to enter the standby state (about 0.5min). Put the flask containing 50ml boric acid solution and 2~3 drops of indicator solution on the receiving tray. The receiving tube is inserted into the liquid surface. Then the digestion tube containing the appropriate amount of digested sample is placed on the distiller, plus 20ml of purified water and when digested. Sulfuric acid 5 times the amount of sodium hydroxide solution, open the steam switch for distillation. When the effluent reaches about 150ml, move the receiving bottle downwards to take the receiving tube off the liquid surface. Rinse the receiving tube with pure water and continue distilling for 0.5 minutes until the end. Remove the digestive tube, turn off the steam switch, and restore the instrument to standby. The distillate was titrated with a 0·0,500 mol/L hydrochloric acid standard solution to lavender and the protein content was calculated. At the same time as reagent blank experiment.
2 nitrogen determination test results
2.1 Method Comparison The protein contents of different types of samples were determined using the classic Kjeldahl method and the KDY-04 (A) nitrogen determination instrument, respectively. There was no significant difference between the two methods in the same sample (t=0.361, P>0.05). (Table 1). 2.2 Determination of the precision of the sample with different protein content, each sample was measured in parallel 6 times, the results are shown in Table 2. The relative standard deviation (RSD) of the results was <2%. 2.2.3 Accuracy Measurement Samples with different protein contents were taken and a certain amount of ammonium sulfate standard was added for the spike recovery test. The recovery was determined to be 97.6% to 101.9%. (table 3). 3 Discussion The classical Kjeldahl method for the determination of protein has complicated equipment, complicated procedures, long digestion time, large environmental pollution, difficult to control the distillation process, and prone to sucking phenomenon resulting in failure of the experiment. The use of the KDY-04 (A) nitrogen determination instrument overcomes the above deficiencies. With closed digestion, the sample is less susceptible to contamination and also eliminates environmental pollution; multiple samples can be digested at one time; no distillation down phenomenon occurs. Improve work efficiency. The appropriate amount of hydrogen peroxide is added during the digestion process to make the reaction more complete and rapid and more thorough digestion. And the instrument is relatively cheap, suitable for basic unit applications. The level of protein in the visible sample can be increased or decreased or the sample volume can be determined. In the absence of catalyst selenium powder, copper sulfate and potassium sulfate can be used as catalysts. 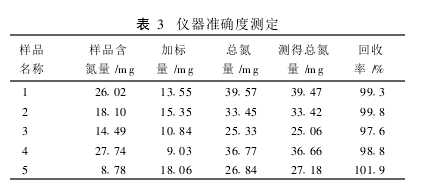
The experimental results showed that KDY-04 (A) determination of protein by nitrogen determination instrument, the determination results are basically consistent with the classic Kjeldahl method, the difference was not statistically significant by t test (t = 0.361, P> 0. 05) . The method is fast, easy to operate, eliminates air pollution, and is suitable for the determination of proteins in batch samples.